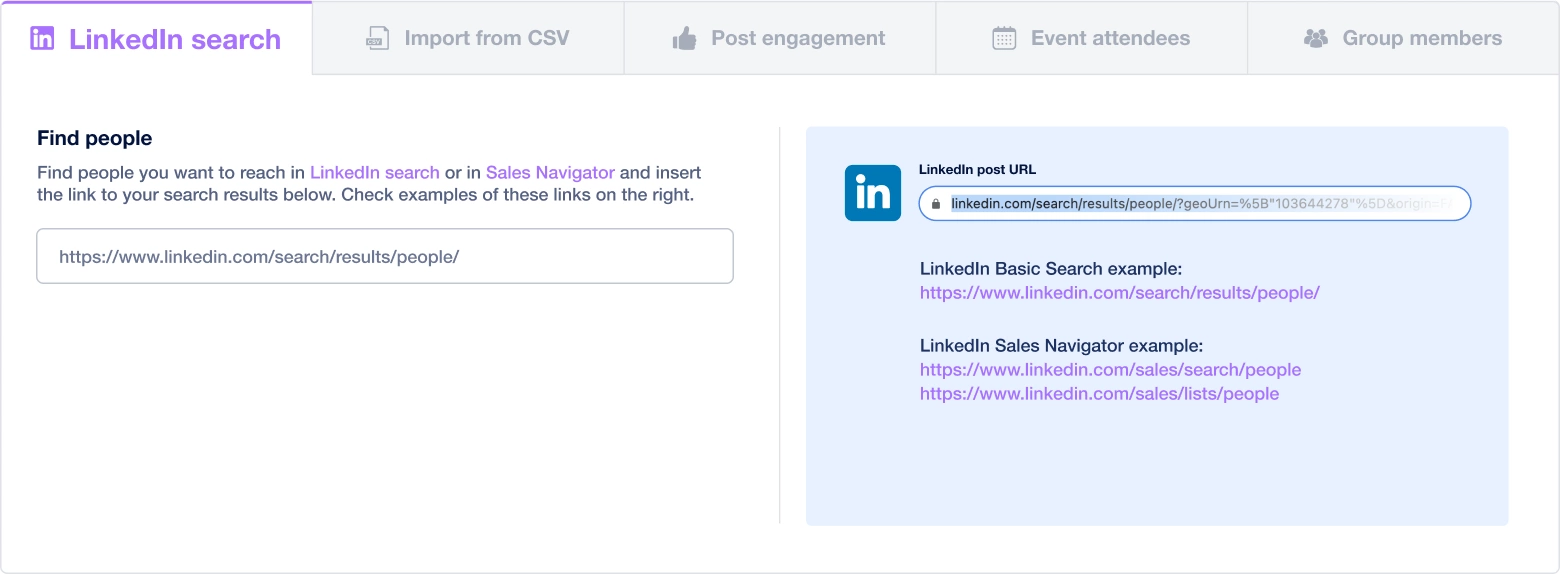
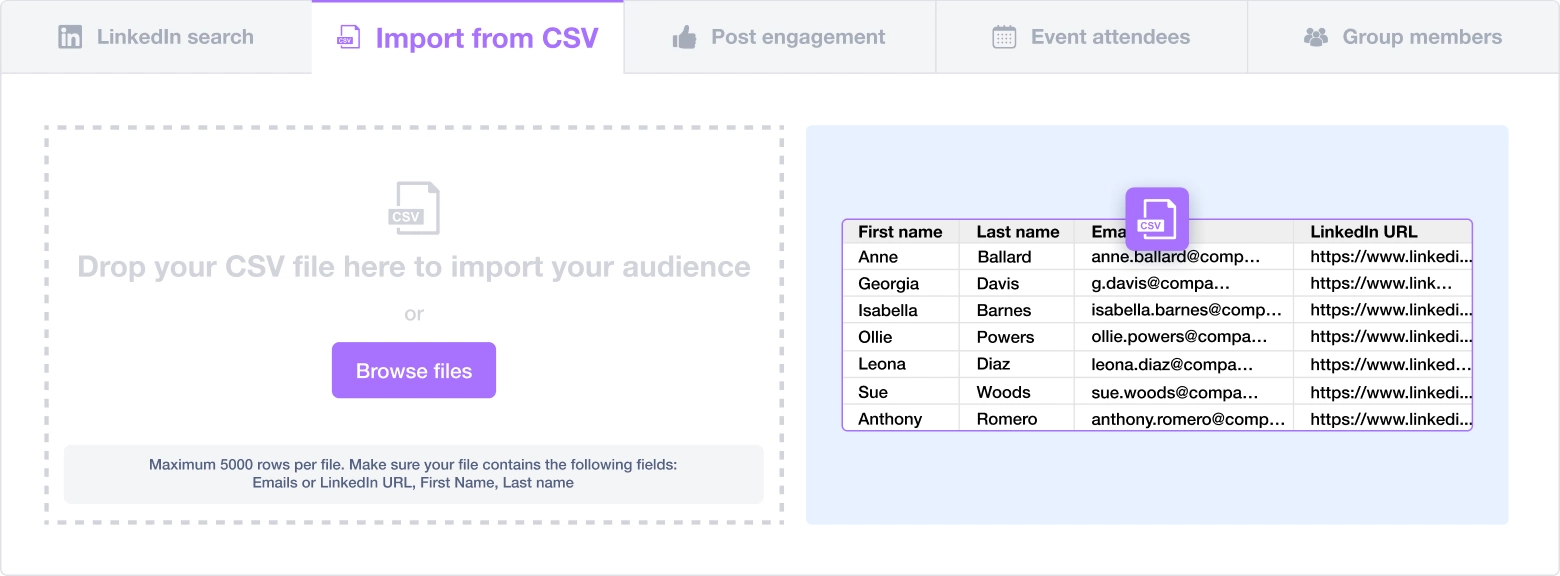
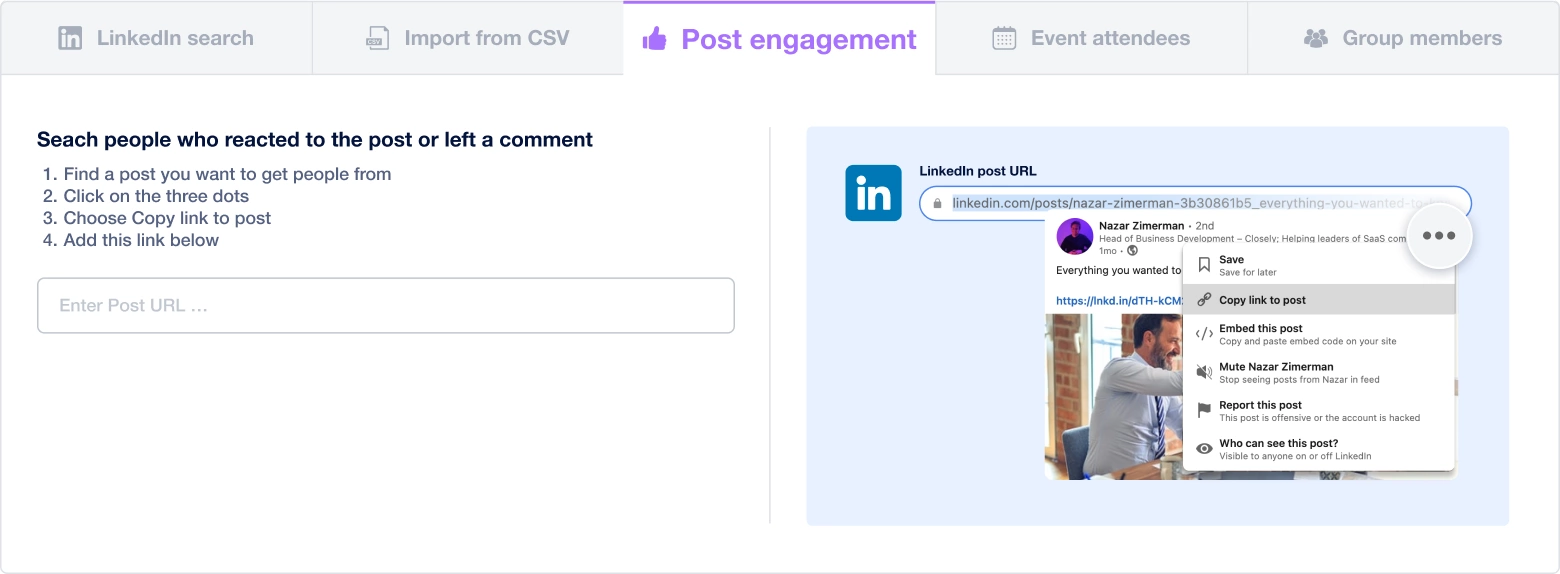
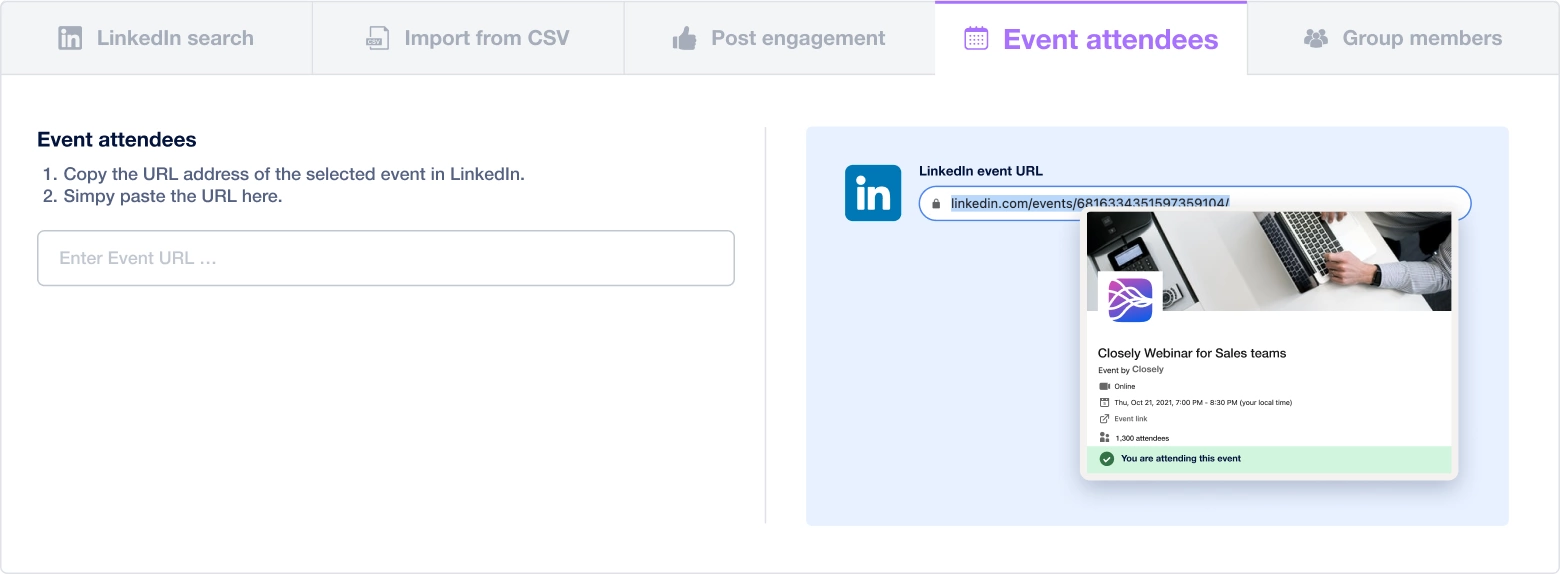
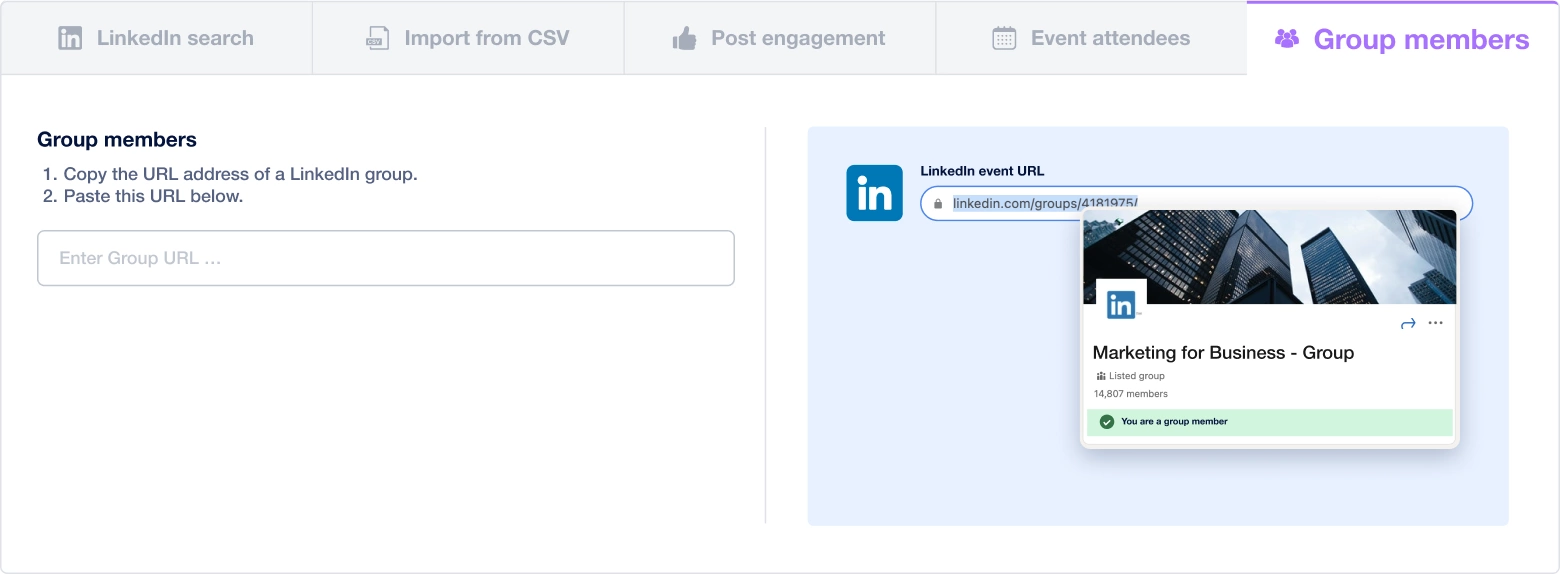
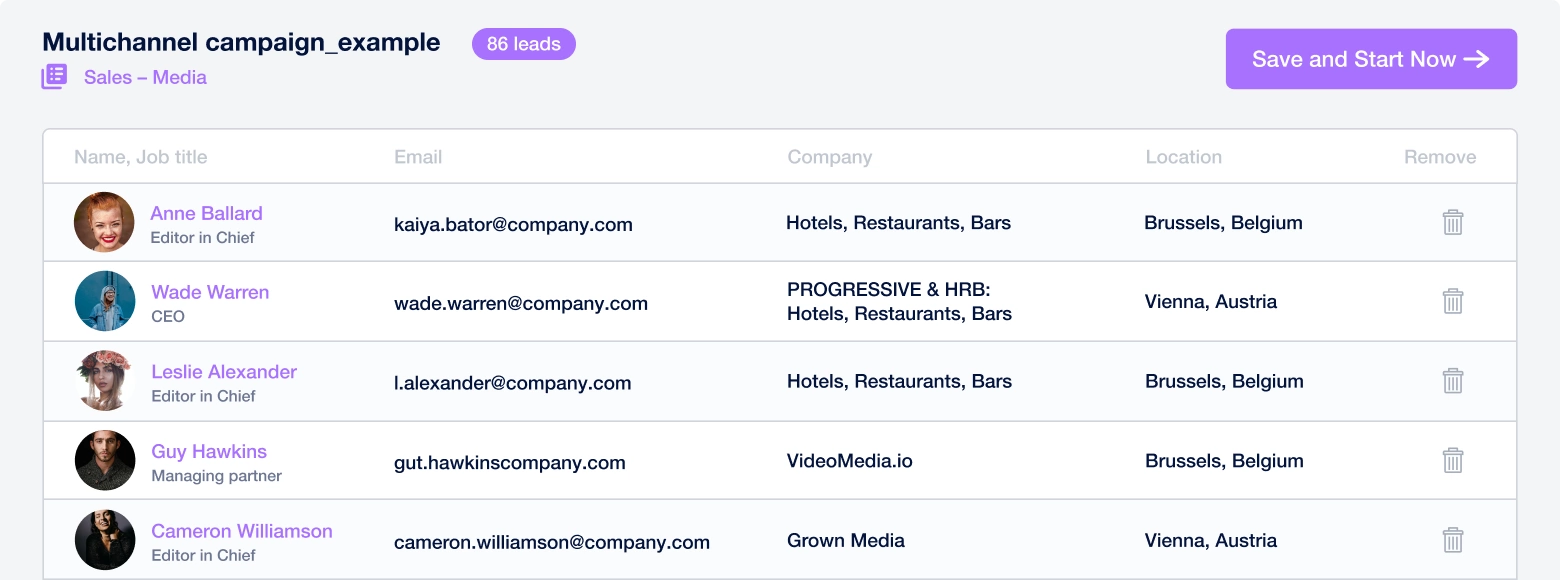
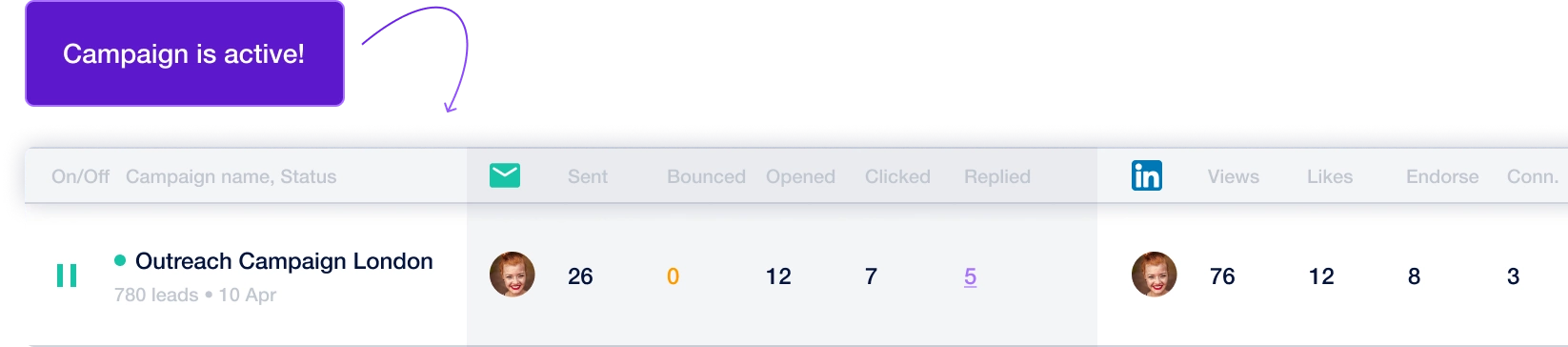
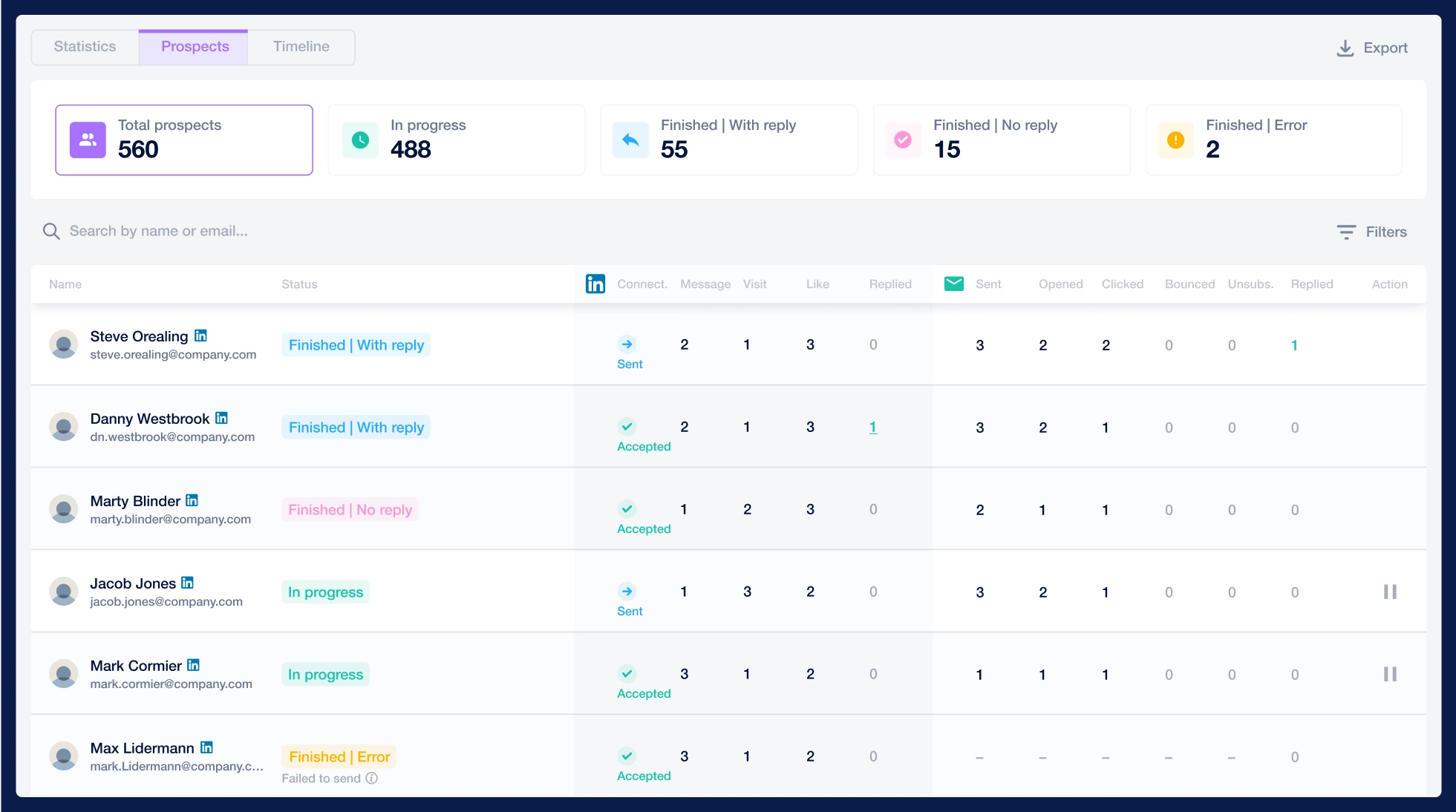
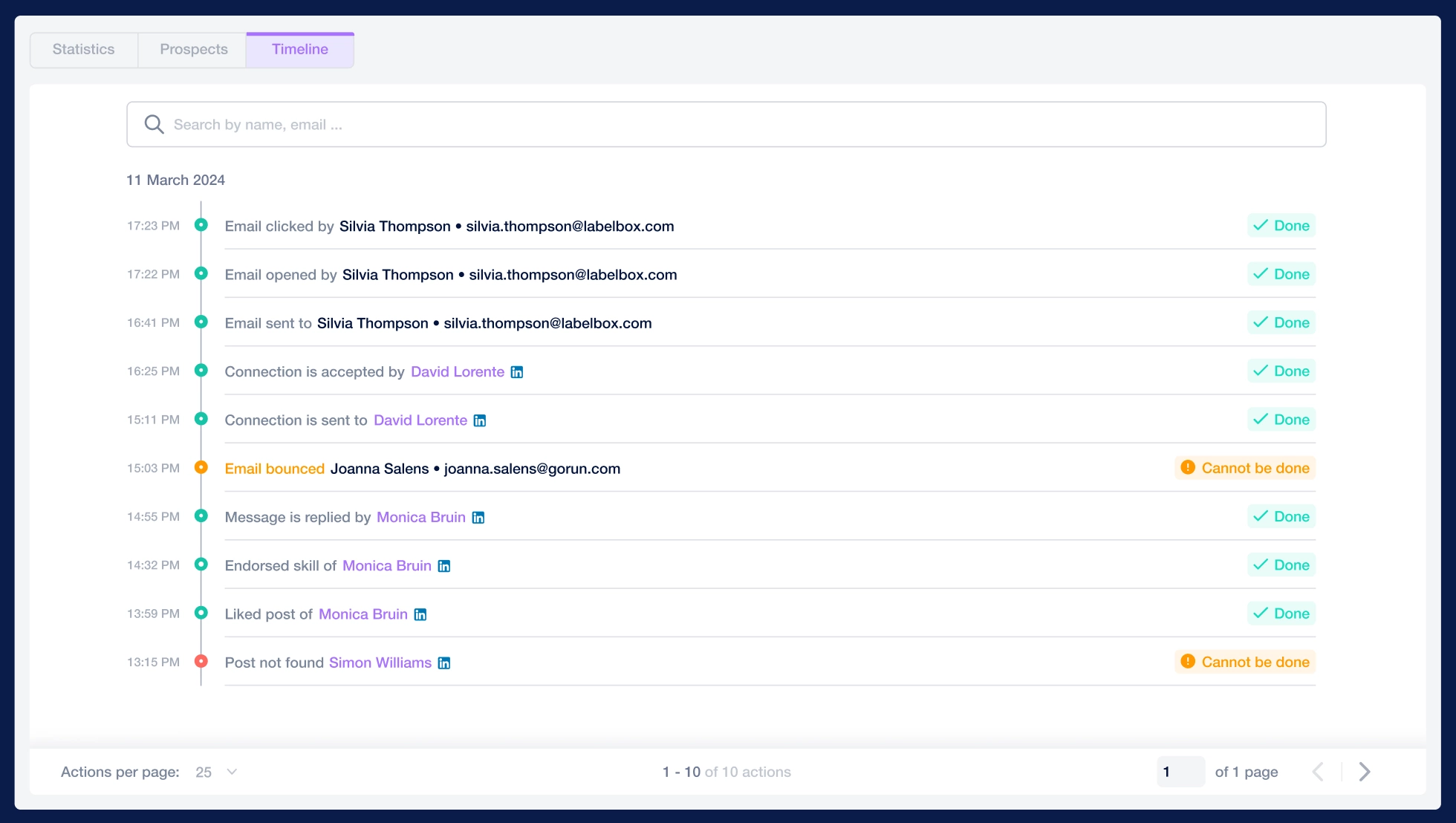
Create a multi-channel campaign
Add actions in LinkedIn or send an email in the sequence you need
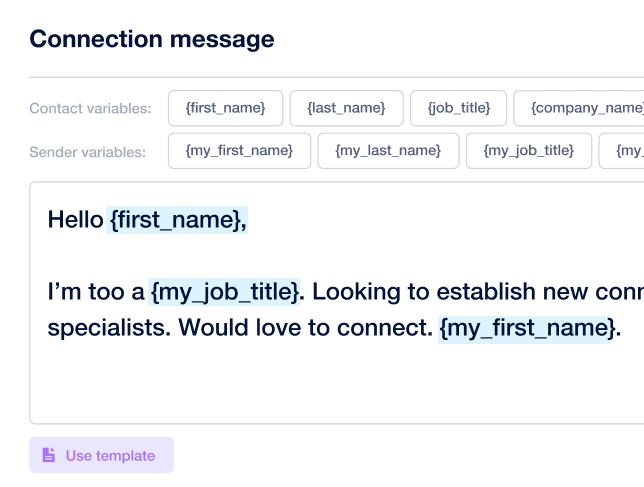
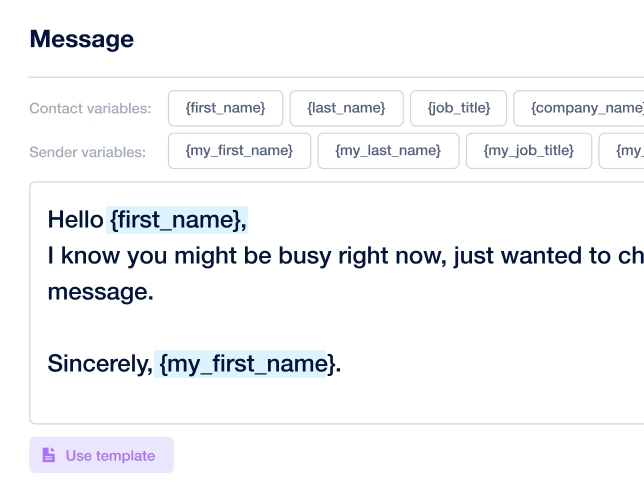
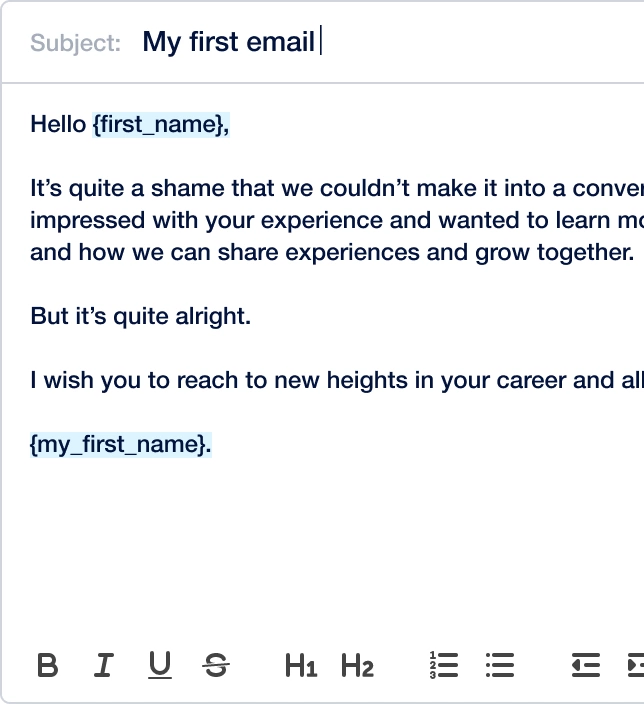
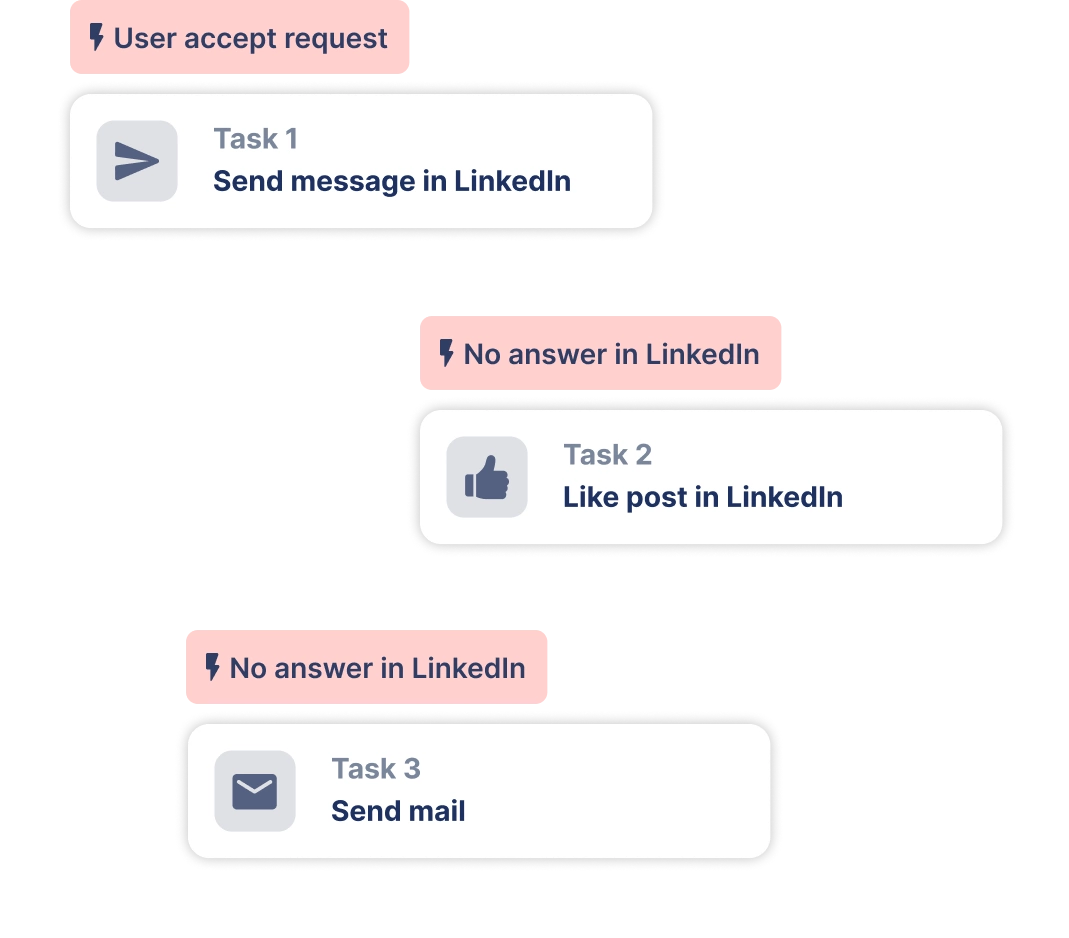
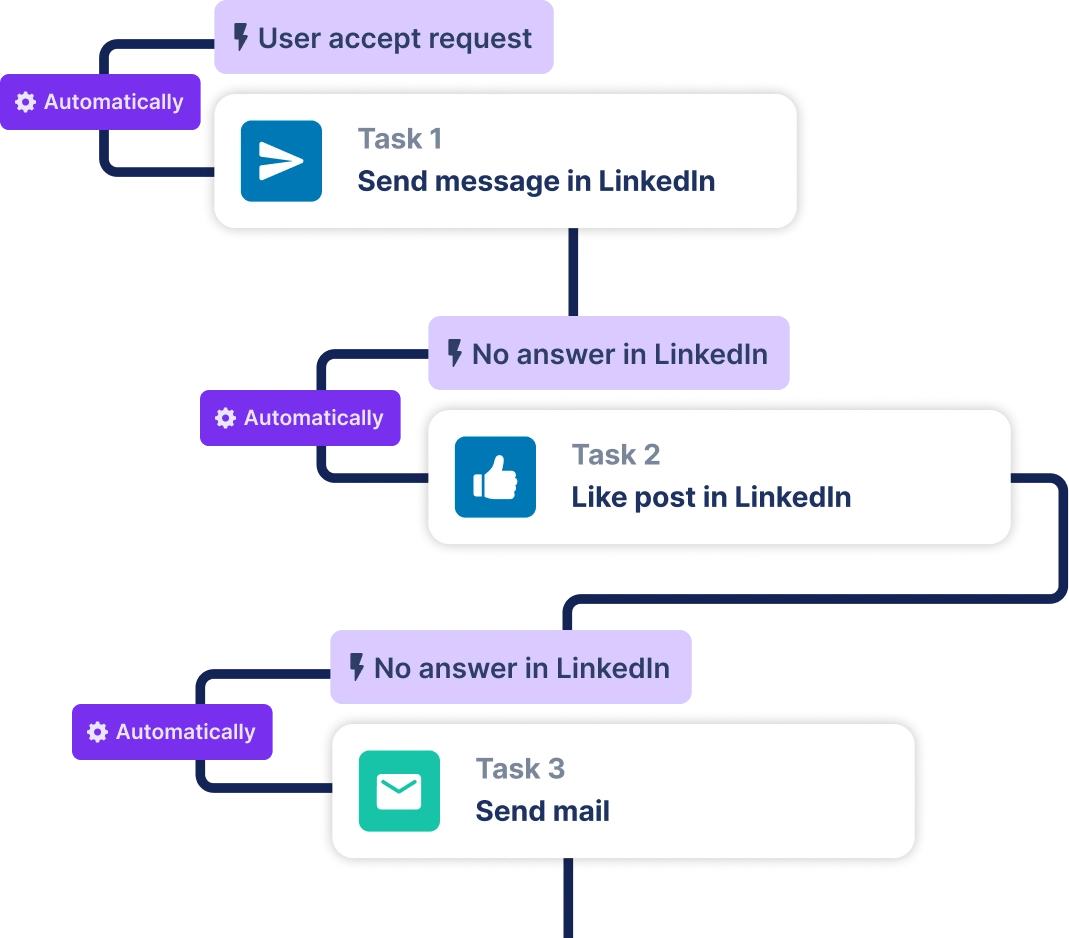
Create outreach campaign in a few clicks!

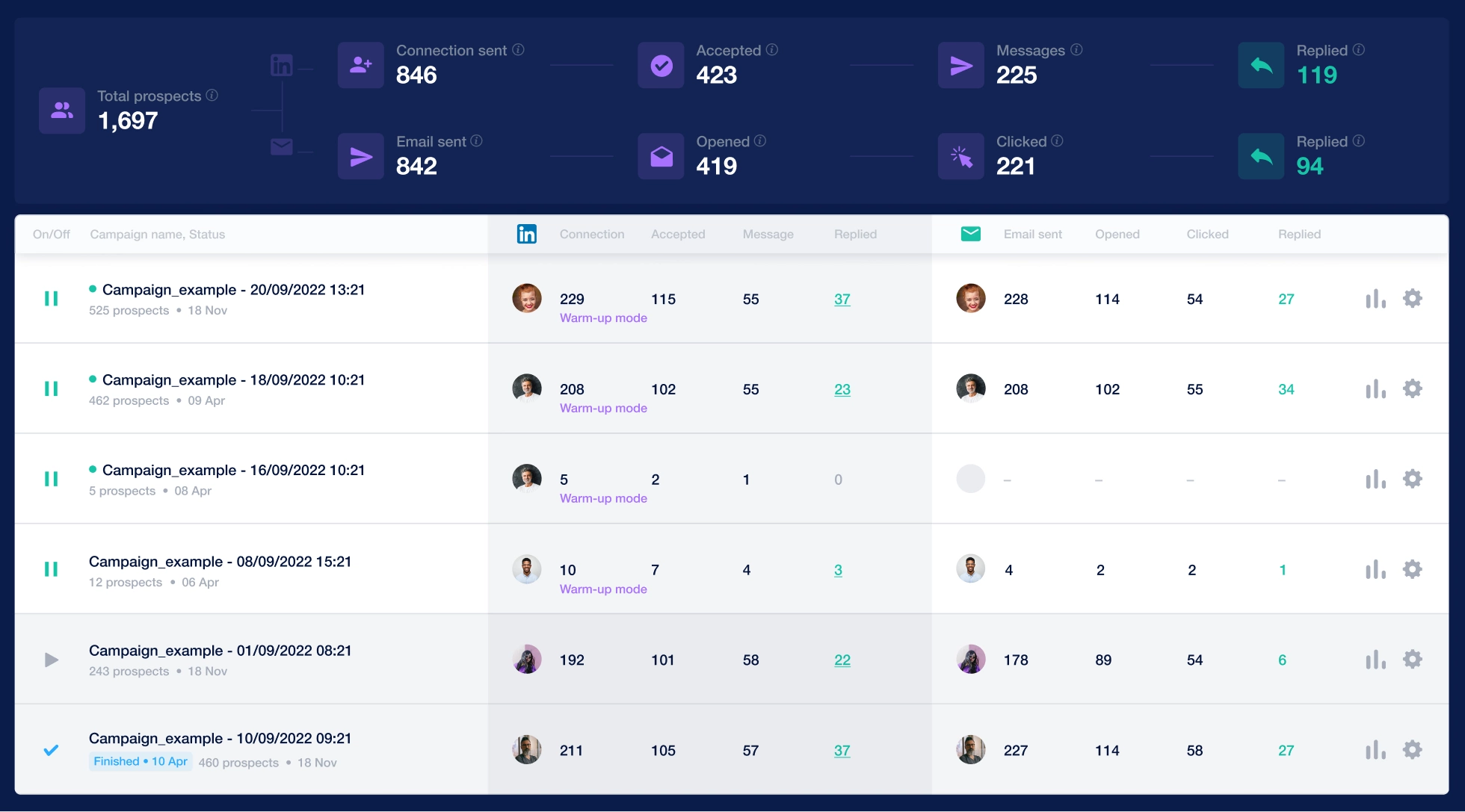




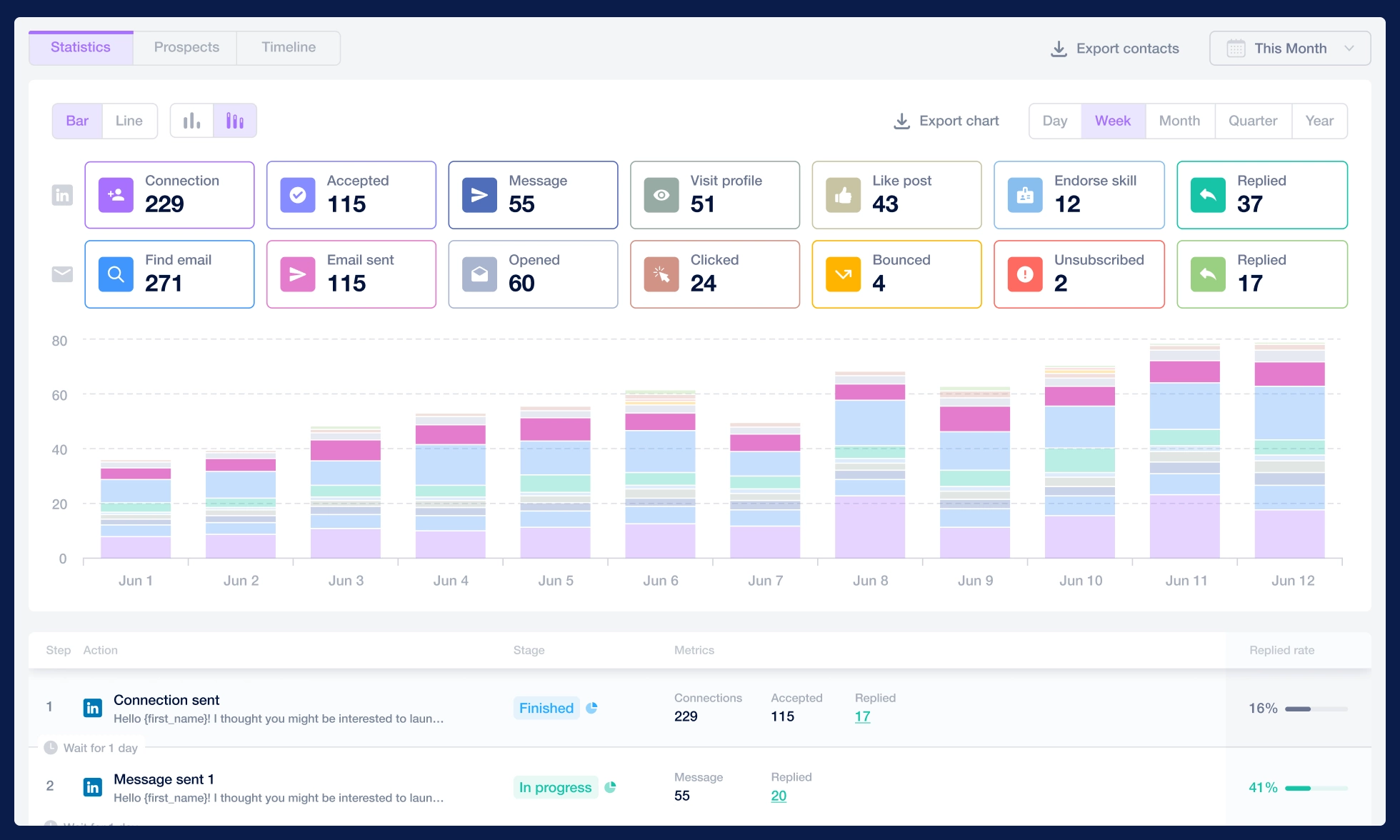

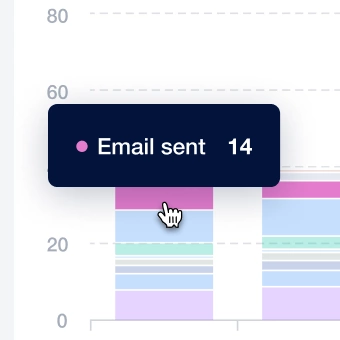



with streamlined tedious tasks: multi-channel letter distribution, advanced analytics, and efficient team management
than alternatives. Elevate your operations with all-in-one platform, harmonizing outreach mail, LinkedIn, AI assistant, and built-in database
decrease in time from prospecting to demo
Closely integrations
Our Ecosystem





Team Management Access
For whom they are?





Pricing
Personal
For individuals
Pro
Appropriate for midsize teams
Closely Results
Closely helps sales teams increase win rates by 22%
more outbound leads
decrease in time from prospecting to demo
increase in demos
Love it!! Ease of use... Workflows are amazing... The support is TOP NOTCH!!
Easy to use and work perfectly for the Team as well - Love chrome extension and automation with it
I really like Closely because you can put different filters and search leads based on your needs, and all this happens in one click. Closely Facilitates LEADS - LinkedIn Engagement And Development System
Love it!! Ease of use... Workflows are amazing... The support is TOP NOTCH!!
Easy to use and work perfectly for the Team as well - Love chrome extension and automation with it
I really like Closely because you can put different filters and search leads based on your needs, and all this happens in one click. Closely Facilitates LEADS - LinkedIn Engagement And Development System